Abstract

Background: tMN is a lethal complication of aPBSCT for Hodgkin (HL) and non-Hodgkin lymphoma (NHL). Clonal somatic mutations in leukemia-associated genes (clonal hematopoiesis [CH]) are observed in peripheral blood of healthy older adults, and are associated with an elevated risk for subsequent leukemia. In cancer patients, CH mutations are differentially selected by therapeutic exposures. We examined the association between CH in the PBSC product and risk of post-aPBSCT tMN.
Methods: We constructed a retrospective cohort of 1,328 patients (NHL=1,000; HL=328) who received aPBSCT between 1980 and 2016 at a single institution. Information on demographics (sex, race), clinical characteristics (HL/NHL, age at aPBSCT, CD34 counts in PBSC product) and therapeutic exposures (pre-aPBSCT cyclophosphamide equivalent dose [CED], doxorubicin equivalence dose, radiation; total body irradiation [TBI]) was abstracted from medical records. Using cryopreserved aliquots of PBSC product from all patients, we performed sequencing of target regions in 91 genes selected for association with CH or myeloid malignancies (Illumina NovaSeq platform; >1000x), to identify pathogenic mutations (variant allele frequency [VAF] ≥1%). Logistic regression identified demographic/clinical/treatment factors associated with CH. Fine-Gray proportional sub-distribution hazard regression determined the role of CH (yes/no; number of mutations [0, 1, 2+]; specific mutations) in development of subsequent tMN, adjusting for above factors. We also examined the role of CH in post-aPBSCT mortality.
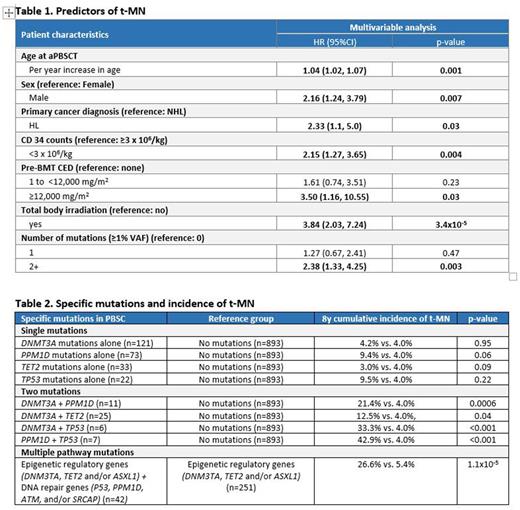
Results: Median age at aPBSCT was 50y (range: 18-78); 62% male; 61% non-Hispanic white. CH in the PBSC was identified in 435 patients (33%); single mutation: 276 (21%); >1 mutation: 159 (12%). Most commonly mutated genes included DNMT3A (192, 14.5%), PPM1D (118, 8.9%), TET2 (82, 6.2%), TP53 (44, 3.3%). Seventy-five patients developed tMN, yielding an 8y cumulative incidence of 5.9%. Predictors for CH in PBSC: Older age at aPBSCT (odds ratio [OR]per_y_inc=1.07, p=5.9x10-34 and high pre-aPBSCT alkylating agent exposure [CED=1-12,000: OR=1.58, p=0.01; CED >12,000: OR=2.03, p=0.057) were independently associated with CH. CH in PBSC and post-aPBSCT t-MN: Among the 435 patients with CH, 38 (9%) developed tMN. The 8y cumulative incidence of tMN was highest in patients with >1 mutations (14.6%) vs. single mutation (6.9%), vs. no mutation (4.0%), p=2.4x10-6. Multivariable analysis (Table 1) revealed >1 mutation as an independent predictor of tMN (HR=2.38, p=0.003; single mutation: HR=1.27, p=0.47; reference: no mutations); other factors associated with tMN are in Table 1. Specific mutations in PBSC andpost-aPBSCTtMN: The role of specific mutations in the development of tMN is detailed in Table 2. tMN incidence in patients with DNMT3A mutations alone did not differ from patients with no mutations (8y incidence: 4.2% vs. 4.0%, p=0.95), but was increased in patients with PPM1D (9.4% vs. 4.0%, p=0.06) and TP53 (9.5% vs. 4.0%, p=0.22) mutations alone, although not reaching statistical significance. The co-occurrence of mutations in DNMT3A + PPM1D (21.4% vs. 4.0%, p=0.0006), DNMT3A +TP53 (33.3% vs. 4.0%, p <0.001) or PPM1D + TP53 (42.9% vs. 4.0%, p <0.001) was associated with significantly higher tMN incidence compared with no CH mutations. Taken together, individuals with the combination of mutations in genes involved in epigenetic regulation (DNM3TA, TET2 and/or ASXL1) and DNA repair (TP53, PPM1D, ATM, and/or SRCAP) had significantly higher tMN incidence compared to those with mutations in genes involved in epigenetic regulation alone (26.6% vs. 5.4%, p=1.1x10-5). Specific mutations at time of tMN: In available samples (peripheral blood or bone marrow: n=40), TP53 (n=13, 32.5%), PPM1D (n=12, 30%), DNMT3A (n=12, 30%) and TET2 (n=4, 10%) were the most frequently mutated genes. CH in PBSC and mortality: All-cause mortality at 8y from aPBSCT was significantly higher in patients with CH (38.4%) vs. those without (30.1%), p=0.0009.
Conclusion and Relevance: CH is associated with increased risk of tMN after aPBSCT for lymphoma, but most patients with CH do not develop tMN. This study shows that the co-occurrence of multiple key somatic mutations in the PBSC product increases tMN risk. Importantly, mutations in DNA repair/ damage response genes, especially in combination with epigenetic regulatory genes, increase the risk of tMN.
Disclosures
Vose:AstraZeneca: Honoraria; Janssen: Honoraria; Lilly: Honoraria; AbbVie: Honoraria; Johnson & Johnson: Consultancy; Daiichi Sankyo: Consultancy; Pharmacyclics: Consultancy; MorphoSys: Consultancy; Kite, a Gilead Company: Research Funding. Weisdorf:FATE Therapeutics: Other: Research Support; Incyte: Other: Research support. Ebert:TenSixteen Bio: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Novartis: Research Funding; GRAIL: Consultancy; Skyhawk Therapeutics: Membership on an entity's Board of Directors or advisory committees; Exo Therapeutics: Membership on an entity's Board of Directors or advisory committees; Deerfield: Research Funding; Celgene: Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.